Products
Important Safety Information | Medication Guide | Full Prescribing Information: Single Dose Vials / Imaging Bulk Package
Gadobutrol Injection
Macrocyclic & High Relaxivity
Gadolinium-Based Contrast Agent3,4
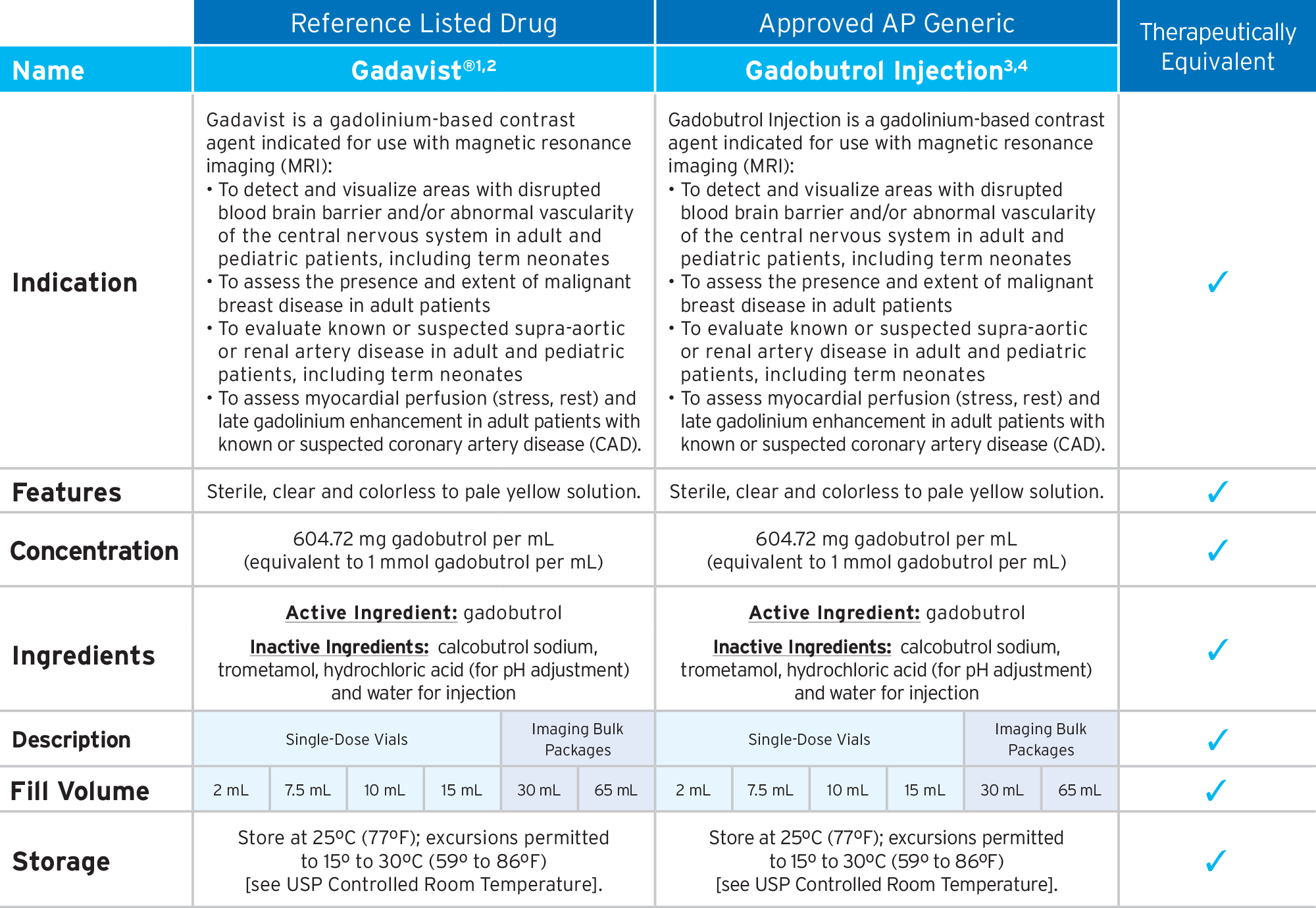
Fresenius Kabi’s FDA-approved generic Gadobutrol Injection provides an option that is bioequivalent and fully substitutable to Gadavist®.*
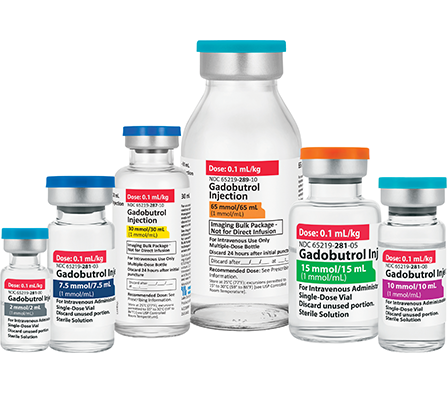
Fresenius Kabi’s Gadobutrol Injection is fully substitutable to Gadavist®.*
Gadobutrol Injection
• FDA-approved, AP Rated
• High Relaxivity3,4
• Macrocyclic Bond3,4
• High Concentration GBCA3,4
• Preservative Free3,4
• The container closure is not made with natural rubber latex
• Bioequivalent and fully substitutable to Gadavist®*
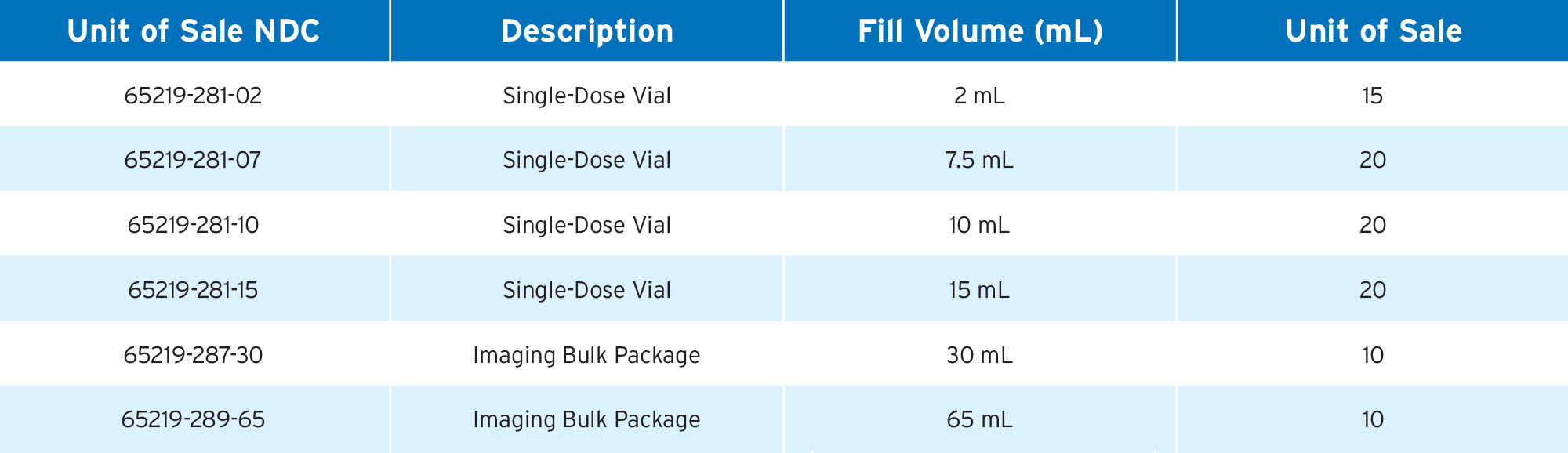
1. Gadavist Single Dose Vial Package Insert, March 2025
2. Gadavist Imaging Bulk Package Insert, March 2025
3. Gadobutrol Injection Single Dose Vial Package Insert, May 2025
4. Gadobutrol Injection Imaging Bulk Package Insert, May 2025
*Gadavist® is a registered trademark of Bayer.
Downloads
Gadobutrol Injection Brochure
Download
Important Safety Information
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. Gadobutrol injection is not approved for intrathecal use.
Nephrogenic Systemic Fibrosis
GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of drugs. Avoid use of gadobutrol injection in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle, and internal organs.
-
The risk for NSF appears highest among patients with:
- Chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
- Acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (for example, age > 60 years, hypertension, or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended gadobutrol injection dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration.
CONTRAINDICATIONS
Gadobutrol Injection is contraindicated in patients with history of severe hypersensitivity reactions to Gadobutrol Injection.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions: Anaphylactic and other hypersensitivity reactions with cardiovascular, respiratory, or cutaneous manifestations, ranging from mild to severe, including death, have uncommonly occurred following gadobutrol administration. Before gadobutrol administration, assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders. These patients may have an increased risk for a hypersensitivity reaction to gadobutrol. Monitor patients closely during and after administration of gadobutrol.
Acute Respiratory Distress Syndrome (ARDS): ARDS has been reported in patients administered gadobutrol injection and may be characterized by severe hypoxemia requiring oxygen support and mechanical ventilation. Onset can occur within <30 minutes to 24 hours after administration. For patients demonstrating respiratory distress after administration, assess oxygen requirement and monitor for worsening respiratory function.
Gadolinium Retention: Gadolinium is retained for months or years in brain, bone, and other organs. Linear GBCAs cause more retention than macrocyclic GBCAs. At equivalent doses, retention varies among the linear agents. Retention is lowest and similar among the macrocyclic GBCAs. Consequences of gadolinium retention in the brain have not been established, but they have been established in the skin and other organs in patients with impaired renal function. While clinical consequences of gadolinium retention have not been established in patients with normal renal function, certain patients might be at higher risk. These include patients requiring multiple lifetime doses, pregnant and pediatric patients, and patients with inflammatory conditions. Consider the retention characteristics of the agent and minimize repetitive GBCA studies, when possible.
Acute Kidney Injury: In patients with chronic renal impairment, acute kidney injury sometimes requiring dialysis has been observed with the use of GBCAs. Do not exceed the recommended dose; the risk of acute kidney injury may increase with higher than recommended doses.
Extravasation and Injection Site Reactions: Ensure catheter and venous patency before the injection of gadobutrol. Extravasation into tissues during gadobutrol administration may result in moderate irritation.
Overestimation of Extent of Malignant Disease in MRI of the Breast: Gadobutrol MRI of the breast overestimated the histologically confirmed extent of malignancy in the diseased breast in up to 50% of the patients.
Low Sensitivity for Significant Arterial Stenosis: The performance of gadobutrol MRA for detecting arterial segments with significant stenosis (>50% renal, >70% supra-aortic) has not been shown to exceed 55%. Therefore, a negative MRA study alone should not be used to rule out significant stenosis.
ADVERSE EVENTS:
The most common adverse reactions (incidence ≥ 0.5%) associated with gadobutrol are headache (1.7%), nausea (1.2%) and dizziness (0.5%).
The following additional adverse reactions have been identified during post marketing use of gadobutrol or other GBCAs:
- Cardiac arrest
- Nephrogenic Systemic Fibrosis (NSF)
- Hypersensitivity reactions (anaphylactic shock, circulatory collapse, respiratory arrest, bronchospasm, cyanosis, oropharyngeal swelling, laryngeal edema, blood pressure increased, chest pain, angioedema, conjunctivitis, hyperhidrosis, cough, sneezing, burning sensation, and pallor)
- Respiratory, Thoracic, and Mediastinal Disorders: Acute respiratory distress syndrome, pulmonary edema
- General Disorders and Administration Site Conditions: Adverse reactions with variable onset and duration have been reported after GBCA administration. These include fatigue, asthenia, pain syndromes, and heterogeneous clusters of symptoms in the neurological, cutaneous, and musculoskeletal systems
- Skin: Gadolinium associated plaques
- Gastrointestinal Disorders: Acute pancreatitis with onset within 48 hours after GBCA administration
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: GBCAs cross the placenta and result in fetal exposure and gadolinium retention. Because of the potential risks of gadolinium to the fetus, use gadobutrol only if imaging is essential during pregnancy and cannot be delayed.
INDICATIONS AND USAGE
Gadobutrol Injection is a gadolinium-based contrast agent indicated for use with magnetic resonance imaging (MRI):
- To detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the central nervous system in adult and pediatric patients (including term neonates).
- To assess the presence and extent of malignant breast disease in adult patients.
- To assess myocardial perfusion (stress, rest) and late gadolinium enhancement in adult patients with known or suspected coronary artery disease (CAD).
Gadobutrol Injection is indicated for use in magnetic resonance angiography (MRA):
- To evaluate known or suspected supra-aortic or renal artery disease in adult and pediatric patients, including term neonates.
This Important Safety Information does not include all the information needed to use Gadobutrol Injection safely and effectively. Please see full prescribing information, including BOXED WARNING, for Gadobutrol Injection Single-Dose Vials and Imaging Bulk Package. Full prescribing information is also available at www.fresenius-kabi.com/us.

